The FDA never inspected Johns Dental Laboratories during more than a decade that it manufactured the Anterior Growth Guidance Appliance, or “AGGA,” a dental device that allegedly harmed patients and is now the subject of a criminal investigation.
According to FDA documents obtained through the Freedom of Information Act, the agency “became aware” of AGGA from a joint investigation by KFF Health News and CBS News in March 2023, and then responded with its first inspection at Johns Dental months later.
Samantha Liss/KFF Health News
That inspection found that the Indiana dental device manufacturer did not require all customer complaints to be investigated and the company did not investigate some complaints about people injured by products, including AGGA, the FDA documents state. The FDA requires device companies investigate complaints and escalate them to the agency. Johns Dental “never” alerted the FDA about such claims, according to the documents.
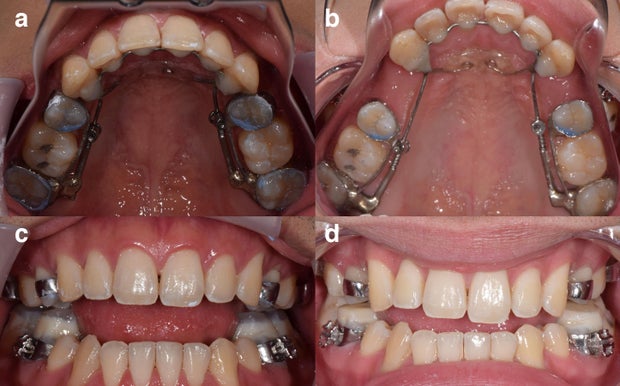
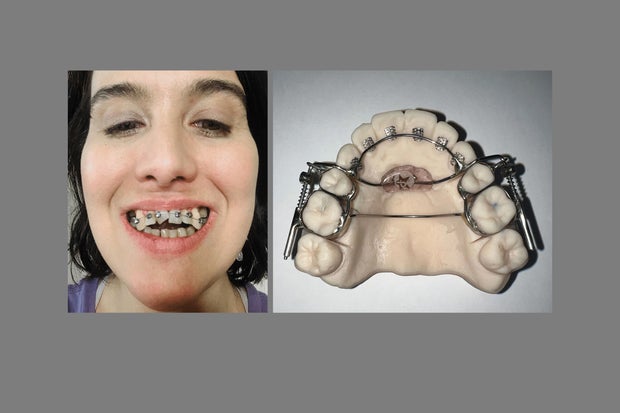
AGGA, whose inventor testified has been used on more than 10,000 patients, was promoted by dentists across the country, some of whom said it could “grow” or “expand” an adult’s jaw without surgery and treat common ailments. like sleep apnea. But these claims were not supported by peer-reviewed research, and Johns Dental settled lawsuits from 20 patients who claimed AGGA caused them serious harm. The company did not admit liability.
Two former FDA officials said AGGA was likely able to stay in business — and off the FDA’s radar — for so long because of a lack of inspections and investigations at Johns Dental. Madris Kinard, former FDA manager who founded Device eventswhich analyzes FDA data, said it challenges the belief that Johns Dental has never received a complaint worthy of being forwarded to the FDA.
“That’s a red flag to me. If I don’t see a single report sent to the FDA, I usually think there’s something going on,” Kinard said. “When they don’t report, what you have are devices that stay on the market much longer than they should. And patients are harmed.”
Johns Dental Laboratories declined to comment when reached by phone and its attorneys did not respond to interview requests. The family-owned company that has operated since 1939 in the western Indiana city of Terre Haute, sells dozens of products to dentists and manufactures hundreds of retainers and sleep apnea devices every month, according to its website.
Twelve of Johns Dental products are registered with the FDA as Class II medical devices, meaning they pose at least a moderate risk, and some have appeared on the company’s website for at least two decades, according to screenshots preserved by the Internet Archive.
Brett Kelman/KHN
The AGGA, which was invented by Tennessee dentist Steve Galella in the 1990s, has not been registered with the FDA like other Johns Dental devices. Company owner Jerry Neuenschwander said in sworn court testimony that Johns Dental began manufacturing AGGA in 2012 and became the exclusive manufacturer of Galella in 2015 and that at one point AGGA accounted for about one-sixth of revenue total sales of Johns Dental.
In another statement, Johns Dental CEO Lisa Bendixen said the company produced about 3,000 to 4,000 AGGAs a year and paid Galella’s company a “royalty” of $50 to $65 for each sale.
“We are not dentists. We don’t know how these devices work. All we do is manufacture them according to Dr. Galella’s specifications,” she said, according to a transcript of the testimony.
The FDA’s lack of knowledge of AGGA likely contributed to its lax oversight of Johns Dental. When asked to explain the lack of inspection, the FDA said that, based on what it knew at the time, it was not required to inspect Johns Dental until 2018, when the company registered as a “contract manufacturer” of other medical devices. Before 2018, the FDA was only aware that Johns Dental operated as a “dental laboratory,” which typically did not manufacture its own products and only modified devices made by other companies to fit dentists’ specifications. The FDA does not regularly inspect dental laboratories, although it may do so if it has questions or receives complaints, the agency said.
Kinard said that based on her experience at the FDA, she believes the agency prioritizes medical devices over dental devices, which may have contributed to the lack of inspections at Johns Dental.
“There hasn’t been a lot of attention to dental devices in the past,” Kinard said. “We hope this changes because of failures in dental implants, as well as this device, which has obviously had serious problems.”
The AGGA resembles a retainer and uses springs to apply pressure to the front teeth and upper palate, according to the patent application. Last year, the KFF Health News-CBS News investigation revealed that AGGA was not supported by any peer-reviewed research and was never submitted to the FDA for review. At the time, at least 20 patients alleged in lawsuits that AGGA had caused severe damage to their teeth, gums and bones — and some said they had lost teeth. Several dental experts said in interviews that they have examined AGGA patients whose teeth were displaced by the device, sometimes causing tens of thousands of dollars in damage.
Kasey Li
“The whole concept of this device, this treatment, doesn’t make any sense at all,” said Kasey Li, a maxillofacial surgeon who published research on AGGA patients which appeared on the National Institutes of Health website. “It doesn’t make the jaw grow. It doesn’t widen the jaw. It just pushes the teeth out of their original position.
Johns Dental and Galella negotiated out-of-court settlements with the original 20 AGGA plaintiffs without publicly admitting guilt. At least 13 more AGGA patients have filed similar lawsuits since the KFF Health News-CBS News investigation. Johns Dental and Galella have denied any wrongdoing or have not yet responded to the allegations in the most recent lawsuits.
Galella declined to be interviewed in 2023 and neither he nor his lawyers responded to recent requests for comment. One of his lawyers, Alan Fumuso, said in a 2023 statement that AGGA “is safe and can achieve beneficial results” when used appropriately.
In the wake of the KFF Health News-CBS News report, Johns Dental abruptly stopped manufacturing AGGA, according to newly released FDA documents. The Department of Justice soon after opened a criminal investigation into AGGA this was in the works in December, according to court documents. No charges were filed. A DOJ spokesperson declined to comment.
Spurred by the March 2023 report, the FDA inspected Johns Dental in July. The FDA website shows that Johns Dental was issued seven citationsbut the substance of the agency’s findings was not known until the inspection report was obtained this year.
FDA investigator David Gasparovich wrote in that report that he arrived unannounced at Johns Dental last July and was met by five lawyers who instructed employees not to answer any questions about AGGA or the company’s complaints policies. Neuenschwander was instructed by his lawyer not to speak to the inspector, the report states.
“He asked if he could photograph my credentials,” Gasparovich wrote in his report. “This was the last conversation I had with Mr. Neuenschwander at the request of his lawyer.”
The FDA requires device companies to investigate product complaints and submit a “medical device report” to the agency within 30 days if the products may have contributed to serious injury or death. Gasparovich’s inspection report states that Johns Dental “did not adequately investigate customer complaints” and its complaint policies were “not adequately established,” allowing employees not to investigate if the product was not first returned to the company.
Boja Kragulj; Claire Miclat
Johns Dental received four complaints about AGGA following the KFF Health News-CBS News report, including one that came after the FDA announced “safety concerns” on the device, according to the inspection report.
“Zero (0) of the four (4) complaints were investigated,” Gasparovich wrote in the report. “Each complaint was closed the same day it was received.”
In the months following Gasparovich’s inspection, Johns Dental sent letters to the FDA saying it had revised its complaints policies to require further investigation and hired a consultant and auditor to address other FDA concerns, according to documents obtained through FOIA. .
Former FDA analyst M. Jason Brooke, now a lawyer who advises medical device companies, said the FDA uses an internal risk-based algorithm to determine when to inspect manufacturers and advises its clients to expect inspections every three to five years.
Brooke said AGGA is an example of how FDA oversight can be undermined by its dependence on transparency from device manufacturers. If device companies don’t report to the agency, it could be unaware of patient complaints, malfunctions or even entire products, he said.
When a company “doesn’t follow the law,” Brooke said, “the FDA is left in the dark.”
“If there are no complaints coming from patients, doctors, competitors or the company itself, then in many ways there is just a dearth of information for the FDA to consume to trigger an inspection,” Brooke said.
CBS News producer Nicole Keller contributed to this report.
KFF Health News is a national newsroom that produces in-depth journalism on health issues and is one of the main operational programs of the KFF – the independent source for health policy research, research and journalism.